Most Offspring Died When Mother Rats Ate Genetically Engineered Soy
By Jeffrey M. Smith
October 29, 2005
The Russian scientist planned a simple experiment to see if eating genetically modified (GM) soy might influence offspring. What she got, however, was an astounding result that may threaten a multi-billion dollar industry.
Irina Ermakova, a leading scientist at the Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Sciences (RAS), added GM soy flour (5-7 grams) to the diet of female rats. Other females were fed non-GM soy or no soy at all. The experimental diet began two weeks before the rats conceived and continued through pregnancy and nursing.
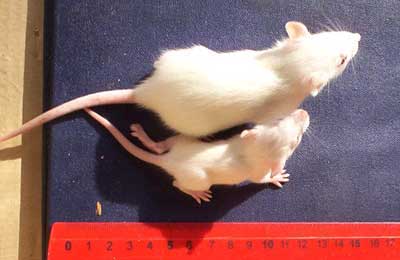
Ermakova's first surprise came when her pregnant rats started giving birth. Some pups from GM-fed mothers were quite a bit smaller. After 2 weeks, 36% of them weighed less than 20 grams compared to about 6% from the other groups (see photo below).
But the real shock came when the rats started dying. Within three weeks, 25 of the 45 (55.6%) rats from the GM soy group died compared to only 3 of 33 (9%) from the non-GM soy group and 3 of 44 (6.8%) from the non-soy controls.
Ermakova preserved several major organs from the mother rats and offspring, drew up designs for a detailed organ analysis, created plans to repeat and expand the feeding trial, and promptly ran out of research money. The $70,000 needed was not expected to arrive for a year. Therefore, when she was invited to present her research at a symposium organized by the National Association for Genetic Security, Ermakova wrote "PRELIMINARY STUDIES" on the top of her paper. She presented it on October 10, 2005 at a session devoted to the risks of GM food.
Her findings are hardly welcome by an industry already steeped in controversy.
GM Soy's Divisive Past
The soy she was testing was Monsanto's Roundup Ready variety. Its DNA has bacterial genes added that allow the soy plant to survive applications of Monsanto's "Roundup" brand herbicide. About 85% of the soy gown in the US is Roundup Ready. Since soy derivatives, including oil, flour and lecithin, are found in the majority of processed foods sold in the US, many Americans eat ingredients derived from Roundup Ready soy everyday.
The FDA does not require any safety tests on genetically modified foods. If Monsanto or other biotech companies declare their foods safe, the agency has no further questions. The rationale for this hands-off position is a sentence in the FDA's 1992 policy that states, "The agency is not aware of any information showing that foods derived by these new methods differ from other foods in any meaningful or uniform way."[1] The statement, it turns out, was deceptive. Documents made public from a lawsuit years later revealed that the FDA's own experts agreed that GM foods are different and might lead to hard-to-detect allergens, toxins, new diseases or nutritional problems. They had urged their superiors to require long-term safety studies, but were ignored. The person in charge of FDA policy was, conveniently, Monsanto's former attorney (and later their vice president). One FDA microbiologist described the GM food policy as "just a political document" without scientific basis, and warned that industry would "not do the tests that they would normally do" since the FDA didn't require any.[2] He was correct.
There have been less than 20 published, peer-reviewed animal feeding safety studies and no human clinical trials—in spite of the fact that millions of people eat GM soy, corn, cotton, or canola daily. There are no adequate tests on "biochemistry, immunology, tissue pathology, gut function, liver function and kidney function,"[3] and animal feeding studies are too short to adequately test for cancer, reproductive problems, or effects in the next generation. This makes Ermakova's research particularly significant. It's the first of its kind.
Past Studies Show Significant Effects
Other studies on Roundup Ready soy also raise serious questions. Research on the liver, the body's major de-toxifier, showed that rats fed GM soy developed misshapen nuclei and other cellular anomalies.[4] This indicates increased metabolic activity, probably resulting from a major insult to that organ. Rats also showed changes in the pancreas, including a huge drop in the production of a major enzyme (alpha-amylase),[5] which could inhibit digestion. Cooked GM soy contains about twice the amount of soy lectin, which can also block nutrient assimilation.[6] And one study showed that GM soy has 12-14% less isoflavones, which are touted as cancer fighting.[7]
An animal feeding study published by Monsanto showed no apparent problems with GM soy,[8] but their research has been severely criticized as rigged to avoid finding problems.[9] Monsanto used mature animals instead of young, more sensitive ones, diluted their GM soy up to 12-fold, used too much protein, never weighed the organs, and had huge variations in starting weights. The study's nutrient comparison between GM and non-GM soy revealed significant differences in the ash, fat, and carbohydrate content, lower levels of protein, a fatty acid, and phenylalanine. Monsanto researchers had actually omitted the most incriminating nutritional differences, which were later discovered and made public. For example, the published paper showed a 27% increase in a known allergen, trypsin inhibitor, while the recovered data raised that to a 3-fold or 7-fold increase, after the soy was cooked. This might explain why soy allergies in the UK skyrocketed by 50% soon after GM soy was introduced.
The gene that is inserted into GM soy produces a protein with two sections that are identical to known allergens. This might also account for the increased allergy rate. Furthermore, the only human feeding trial ever conducted confirmed that this inserted gene transfers into the DNA of bacteria inside the intestines. This means that long after you decide to stop eating GM soy, your own gut bacteria may still be producing this potentially allergenic protein inside your digestive tract.
The migration of genes might influence offspring. German scientists found fragments of the DNA fed to pregnant mice in the brains of their newborn.[10] Fragments of genetically modified DNA were also found in the blood, spleen, liver and kidneys of piglets that were fed GM corn.[11] It was not clear if the GM genes actually entered the DNA of the animal, but scientists speculate that if it were to integrate into the sex organ cells, it might impact offspring.
The health of newborns might also be affected by toxins, allergens, or anti-nutrients in the mother's diet. These may be created in GM crops, due to unpredictable alterations in their DNA. The process of gene insertion can delete one or more of the DNA's own natural genes, scramble them, turn them off, or permanently turn them on. It can also change the expression levels of hundreds of genes. And growing the transformed cell into a GM plant through a process called tissue culture can create hundreds or thousands of additional mutations throughout the DNA.
Most of these possibilities have not been properly evaluated in Roundup Ready soy. We don't know how many mutations or altered gene expressions are found in its DNA. Years after it was marketed, however, scientists did discover a section of natural soy DNA that was scrambled[12] and two additional fragments of the foreign gene that had escaped Monsanto's detection.
Those familiar with the body of GM safety studies are often astounded by their superficiality. Moreover, several scientists who discovered incriminating evidence or even expressed concerns about the technology have been fired, threatened, stripped of responsibilities, or censured.[13] And when problems do arise, they are not followed up. For example, animals fed GM crops developed potentially precancerous cell growth, smaller brains, livers and testicles, damaged immune systems, bigger livers, partial atrophy of the liver, lesions in the livers, stomachs, and kidneys, inflammation of the kidneys, problems with their blood cells, higher blood sugar levels, and unexplained increases in the death rate. (See Spilling the Beans, August 2004.) None have been adequately followed-up or accounted for.
Ermakova's research, however, will likely change that. That's because her study is easy to repeat and its results are so extreme. A 55.6% mortality rate is enormous and very worrisome. Repeating the study is the only reasonable option.
American Academy of Environmental Medicine Urges NIH to Follow Up Study
I presented Dr. Ermakova's findings, with her permission, at the annual conference of the American Academy of Environmental Medicine (AAEM) in Tucson on October 27, 2005. In response, the AAEM board passed a resolution asking the US National Institutes of Health (NIH) to sponsor an immediate, independent follow-up of the study. Dr. Jim Willoughby, the Academy's president, said, "Genetically modified soy, corn, canola, and cottonseed oil are being consumed daily by a significant proportion of our population. We need rigorous, independent and long-term studies to evaluate if these foods put the population at risk."
Unfortunately, there is a feature about GM crops that makes even follow-up studies a problem. In 2003, a French laboratory analyzed the inserted genes in five GM varieties, including Roundup Ready soybeans.[14] In each case, the genetic sequence was different than that which had been described by the biotech companies years earlier. Had all the companies made a mistake? That's unlikely. Rather, the inserted genes probably rearranged over time. A Brussels lab confirmed that the genetic sequences were different than what was originally listed. But the sequences discovered in Brussels didn't all match those found by the French.[15] This suggests that the inserted genes are unstable and can change in different ways. It also means that they are creating new proteins—ones that were never intended or tested. The Roundup Ready soybeans used in the Russian test may therefore be quite different from the Roundup Ready soybeans used in follow-up studies.
Unstable genes make accurate safety testing impossible. It also may explain some of the many problems reported about GM foods. For example, nearly 25 farmers in the US and Canada say that certain GM corn varieties caused their pigs to become sterile, have false pregnancies, or give birth to bags of water. A farmer in Germany claims that a certain variety of GM corn killed 12 of his cows and caused others to fall sick. And Filipinos living next to a GM cornfield developed skin, respiratory, and intestinal symptoms and fever, while the corn was pollinating. The mysterious symptoms returned the following year, also during pollination, and blood tests on 39 of the Filipinos showed an immune response to the Bt toxin—created by the GM corn.
These problems may be due to particular GM varieties, or they may result from a GM crop that has "gone bad" due to genetic rearrangements. Even GM plants with identical gene sequences, however, might act differently. The amount of Bt toxin in the Philippine corn study described above, for example, varied considerably from kernel to kernel, even in the same plant.[16]
With billions of dollars invested in GM foods, no adverse finding has yet been sufficient to reverse the industry's growth in the US. It may take some dramatic, indisputable, and life-threatening discovery. That is why Ermakova's findings are so important. If the study holds up, it may topple the GM food industry.
I urge the NIH to agree to the AAEM's request, and fund an immediate, independent follow-up study. If NIH funding is not forthcoming, our Institute for Responsible Technology will try to raise the money. This is not the time to wait. There is too much at stake.
Jeffrey M. Smith is working with a team of international scientists to catalog all known health risks of GM foods. He is the author of Seeds of Deception , the world's bestselling book on GM food, and the producer of the video, Hidden Dangers in Kids' Meals.
References
- "Statement of Policy: Foods Derived from New Plant Varieties," Federal Register vol. 57, no. 104 at 22991, May 29, 1992
- Louis J. Pribyl, "Biotechnology Draft Document, 2/27/92," March 6, 1992, www.biointegrity.org
- Epidemiologist Judy Carman's testimony before New Zealand's Royal Commission of Inquiry on Genetic Modification, 2001.
- Malatesta M, Caporaloni C, Gavaudan S, Rocchi MB, Serafini S, Tiberi C, Gazzanelli G. (2002a) Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell Struct Funct. 27: 173-180.
- Manuela Malatesta, et al, Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean, Journal of Anatomy, Volume 201 Issue 5 Page 409 - November 2002
- Stephen R. Padgette and others, "The Composition of Glyphosate-Tolerant Soybean Seeds Is Equivalent to That of Conventional Soybeans," The Journal of Nutrition, vol. 126, no. 4, April 1996 (The data was taken from the journal archives, as it had been omitted from the published study.)
- Lappe, M.A., Bailey, E.B., Childress, C. and Setchell, K.D.R. (1999) Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. Journal of Medical Food 1, 241-245.
- Stephen R. Padgette and others, "The Composition of Glyphosate-Tolerant Soybean Seeds Is Equivalent to That of Conventional Soybeans," The Journal of Nutrition, vol. 126, no. 4, April 1996
- For example, Ian F. Pryme and Rolf Lembcke, "In Vivo Studies on Possible Health Consequences of genetically modified food and Feed—with Particular Regard to Ingredients Consisting of Genetically Modified Plant Materials," Nutrition and Health, vol. 17, 2003
- Doerfler W; Schubbert R, "Uptake of foreign DNA from the environment: the gastrointestinal tract and the placenta as portals of entry," Journal of molecular genetics and genetics Vol 242: 495-504, 1994
- Raffaele Mazza1, et al, "Assessing the Transfer of Genetically Modified DNA from Feed to Animal Tissues," Transgenic Research, October 2005, Volume 14, Number 5, pp 775 - 784
- P. Windels, I. Taverniers, A. Depicker, E. Van Bockstaele, and M. DeLoose, "Characterisation of the Roundup Ready soybean insert," European Food Research and Technology, vol. 213, 2001, pp. 107-112
- Jeffrey M. Smith, Seeds of Deception, Yes! Books, 2003
- Collonier C, Berthier G, Boyer F, Duplan M-N, Fernandez S, Kebdani N, Kobilinsky A, Romanuk M, Bertheau Y. Characterization of commercial GMO inserts: a source of useful material to study genome fluidity. Poster presented at ICPMB: International Congress for Plant Molecular Biology (n°VII), Barcelona, 23-28th June 2003. Poster courtesy of Dr. Gilles-Eric Seralini, Président du Conseil Scientifique du CRII-GEN, www.crii-gen.org; also "Transgenic lines proven unstable" by Mae-Wan Ho, ISIS Report, 23 October 2003 www.i-sis.org.uk
- http://www.i-sis.org.uk/UTLI.php
- http://www.seedsofdeception.com/utility/showArticle/?objectID=36